Johns Hopkins Team Develops Method to Make Dialysis Fluid for Patients with COVID-19
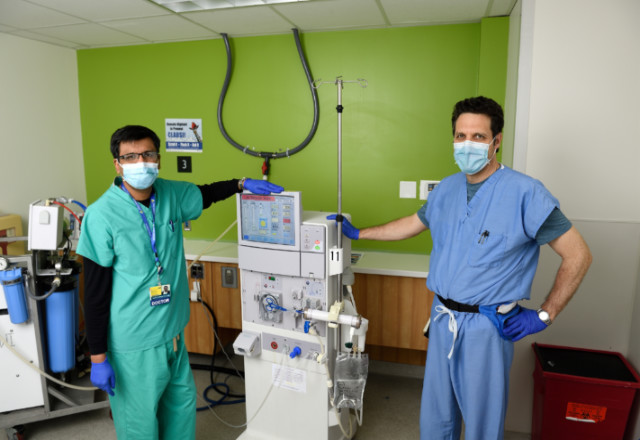
Nephrologists Chirag Parikh (left) and Derek Fine manipulated a conventional dialysis machine (shown here) to produce dialysis fluid for continuous veno-venous hemodialysis, the type of dialysis used in intensive care.
On April 9, clinicians from two New York-based hospitals contacted Derek Fine, clinical director of nephrology at the Johns Hopkins University School of Medicine. “They said they were running out of dialysis fluid and asked if we could spare some from our supply,” says Chirag Parikh, director of the Division of Nephrology at the Johns Hopkins University School of Medicine. “As the severity of COVID-19 increases and patients are on ventilators for a long time, their needs for dialysis treatment to preserve the function of their kidneys also increases.”
In less than two weeks, Fine and Parikh assembled a team of nephrologists, nurses and technicians from the Division of Nephrology and students from the Johns Hopkins Department of Biomedical Engineering to develop a method to make dialysis fluid for the type of dialysis used in intensive care: continuous veno-venous hemodialysis (CVVHD).
Kidney Injury Among Critically Ill Patients with COVID-19
According to the National Kidney Foundation, approximately 3% to 9% of patients with COVID-19 develop an acute kidney injury, with many requiring dialysis. The reason is unknown, but acute kidney injury is often caused by an infection or other serious illness and can occur in a matter of hours or days. When this happens, the kidneys are unable to remove waste and excess fluids from the blood as they normally do.
In urgent situations, such as in the intensive care unit, a central venous catheter is placed in a large vein to enable access to the person’s blood for dialysis treatments. One tube from the catheter carries blood to a dialysis machine and another tube returns processed blood to the patient.
Conventional Dialysis Versus Dialysis in Intensive Care
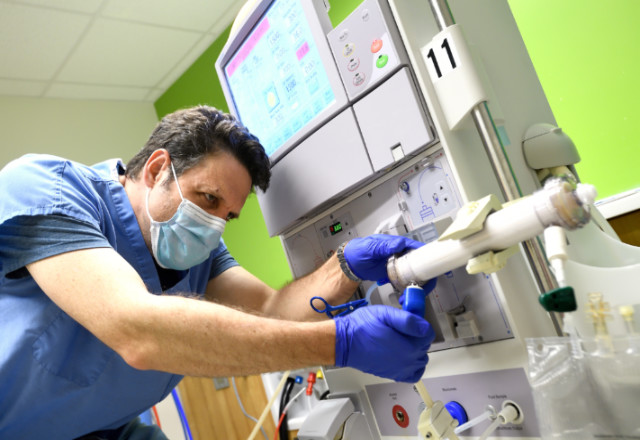
The dialysis machine has an important filter called a dialyzer — also known as an artificial kidney — that has two compartments separated by a thin membrane. While blood is pumped through one side, dialysis fluid — called dialysate — is pumped through the other side. The dialysate attracts waste and excess fluid through the membrane and out of the blood. The filtered blood is then pumped back into the patient, and the used dialysate drains out of the dialyzer.
Unlike conventional dialysis that takes place in an outpatient setting several times per week to treat chronic kidney conditions, CVVHD is slowly administered 24 hours each day for patients who “need a much gentler form of dialysis that doesn’t put stress on their heart and blood pressure while their kidneys recover,” says Parikh.
The machines employed for dialysis in intensive care use pre-mixed, commercially available bags of dialysate. This contrasts with conventional dialysis machines, which generate dialysate in real time for each treatment using ultrapure water and concentrated chemical solutions.
A Shortage of Dialysate, an Abundance of Determination

When Parikh learned that the New York-based hospitals needed 3,000 liters of dialysate per day, he and Fine wondered if they could make dialysate for CVVHD with a conventional dialysis machine. “We did not have enough dialysis fluid on hand to support them,” says Parikh. “The amount they needed for one day was roughly what our hospital requires for one week.”
The nephrologists called on the expertise of the nurses and technicians from the Division of Nephrology to learn the ins and outs of a conventional dialysis machine, and how to make adjustments to override alarms. “All sorts of alarms go off when the machine is being used in an unexpected way,” says Fine. “You have to bypass the alarms because the machine won’t run when an alarm sounds.”
Once they manipulated the machine to work the way they wanted, they needed to collect the dialysate it produced. Fine identified sterile bags used for intravenous nutrition at the hospital as the best choice — but then they needed a way to fasten the bag to the dialyzer.
Parikh contacted students from the Department of Biomedical Engineering to devise a way to attach the bag to the opening on the dialyzer where the used dialysate usually exits the machine. Twelve hours later, the students had designed a connector and used a 3D printer to render the plastic piece. “When we tried it out, we were successfully able to capture the dialysate, and that was the eureka moment,” says Parikh.
The final step was to test the quality of the dialysate. Over a 24-hour period, Fine and Parikh designed a detailed protocol, and sampled the fluid’s chemical composition and looked for bacteria growth. The composition remained stable and they found no bacteria.
Instructions and Guidelines for Making Dialysis Fluid
On April 20, the team shared its method with experts at the U.S. Food and Drug Administration, which provided guidelines for using the method. These guidelines recommend using the dialysate within 12 hours of making it, and testing the dialysate intermittently to ensure it remains bacteria free.
Parikh shared the method with the clinicians in New York, who reported they were able to produce and collect dialysate for CVVHD using the technique. For other hospitals with a shortage of dialysate, instructions to convert a conventional dialysis machine and the files for printing the connector are available in a Google document or on Twitter from @KidneydrChirag.
“Alone, none of us could have done this so quickly,” says Fine. “Fortunately, we were able to assemble a successful team on very short notice.”